ESDEP WG 18
STAINLESS STEEL
To discuss the mechanisms of corrosion in stainless steel and give guidance on selecting the appropriate grade for the application. Guidance on good detailing practice to avoid corrosion is also given.
Lecture 18.1: Introduction to Stainless Steel
Lecture 18.2: Structural Behaviour and Design
Lecture 18.4: Fabrication
This lecture describes why stainless steel may corrode, the mechanisms of corrosion in stainless steel and grade selection to avoid corrosion. Guidance is also given on good detailing practice, storage and handling to obtain the best properties of stainless steel, i.e. good aesthetics and stability of its appearance throughout.
All common structural metals form surface oxide films when exposed to dry air. The oxide formed on mild steel is readily broken down, and in the presence of moisture, it is not repaired. Thus, a reaction can take place between the steel (Fe), the moisture (H2O) and oxygen (O2) to form rust. The reaction is complex but can be represented by a chemical equation of the form:
4 Fe + 2 H2O + 3 O2 = 2 Fe2O3.H2O
Fe2O3.H2O is what is commonly known as rust and, as it is not usually protective, the corrosion process is not impeded.
An oxide is also formed on stainless steel. It consists of a chromium-rich oxide which is stable, non-porous and tightly adherent to the metal. However, unlike that formed on mild steel, if it is broken down (e.g. by scratching or cutting), it is capable of self repair in the presence of air or an oxidising environment. It is also highly resistant to chemical attack. For these reasons it is known as a "passive film". Although the film is very thin (approximately 10-6mm), it gives stainless steel its high corrosion resistance properties by preventing the steel from reacting with the atmosphere.
The behaviour of the passive film depends on the composition of the steel, its surface treatment and the corrosive nature of its environment. The stability of the layer increases as the chromium content increases, that is, nickel that is added for making steel working easier also decreases the corrosion rate. To enhance their corrosion resistance, some stainless steels are "low carbon content" or are stabilised by additions of titanium and niobium, others contain extra molybdenum.
The concept of passive film formation is important because any conditions which prevent the formation of the film or cause it to break down will also lead to loss of corrosion resistance. Corrosion in stainless steel therefore occurs if the passive film is damaged and is not allowed to re-form.
This lecture describes why stainless steel may corrode, the mechanisms of corrosion in stainless steel and grade selection to avoid corrosion. Guidance is also given on good detailing practice, storage and handling to improve corrosion resistance. Further guidance can be found in [1].
Stainless steels are generally very corrosion resistant and will perform satisfactorily in most environments. The limit of corrosion resistance of a given stainless steel depends on its alloying elements which means that each grade has a slightly different response when exposed to a corrosive environment. Care is therefore needed to select the most appropriate grade of stainless steel for a given application. Generally, the higher the level of corrosion resistance required, the higher the level of alloying elements and the greater the cost of the material.
The most common reasons for a metal to fail to live up to expectations regarding corrosion resistance are:
a) incorrect assessment of the environment or exposure to unexpected conditions, e.g. unsuspected contamination by chloride ions.
b) the way in which the stainless steel has been worked or treated may introduce a state not envisaged in the initial assessment.
Although stainless steels may be subject to discolouration and staining (often due to carbon steel contamination), they are extremely durable in buildings. In aggressive industrial and marine environments, tests have shown no indication of reduction in component resistance even where a small amount of weight loss occurred. However, unsightly rust staining on external surfaces may still be regarded as a failure by the user. Experience indicates that any serious corrosion problem is most likely to show up in the first two or three years of service; problems can usually be explained by defects in bringing stainless steels into operation. Except in the case of bad choice of grade, stainless steel can be "cleaned" and gives good results on a very large lifetime. For instance, CHRYSLER BUILDING ROOF in New York made in 1929 and cleaned in the 80's is still in perfect condition.
In certain aggressive environments some grades of stainless steel are susceptible to localised attack. Six mechanisms are described below, although the last three are very rarely encountered in buildings onshore.
Pitting is a localised form of corrosion which can occur in wet conditions as a result of exposure to specific environments, most notably those containing chlorides. Pitting occurs because chloride ions penetrate the passive film in weak spots. A local element is formed with the penetrated area as the anode and the surrounding passive film as the cathode. Since the anode area is small and the cathode area large, the current density, and thereby the corrosion rate on the anode surface becomes very high. Pitting needs wet conditions; it stops when surface is dried by sun and cannot appear if surface is kept dry.
In most structural applications, the extent of pitting is likely to be superficial and the reduction in section of a component is negligible. However, corrosion products can stain architectural features. A less tolerant view of pitting should be adopted for services such as ducts, piping and containment structures. If there is a known pitting hazard, then a molybdenum bearing stainless steel will be required.
Crevice corrosion is a localised form of attack which is initiated by the extremely low availability of oxygen in a crevice. It is only likely to be a problem in stagnant solutions where a build-up of chlorides can occur. The severity of crevice corrosion is very dependent on the geometry of the crevice; the narrower and deeper the crevice, the more severe the corrosion. Crevices typically occur between nuts and washers or around the thread of a screw or the shank of a bolt. Crevices can also occur in welds which fail to penetrate and under deposits on the steel surface. To avoid crevice corrosion under deposits, periodic cleaning is recommended; washing by rain helps considerably; however, periodic cleaning should be part of the building maintenance. To avoid crevice corrosion under bolts etc., it is recommended to avoid water penetration by use of plastics or rubber gaskets, or by filling crevices with adherent sealant or mastic.
Bimetallic (galvanic) corrosion may occur when dissimilar metals are in electrical contact in a common electrolyte (e.g. rain, condensation etc.). If current flows between the two, the less noble metal (the anode) corrodes at a faster rate than would have occurred if the metals were not in contact.
The rate of corrosion also depends on the relative areas of the metals in contact, the temperature and the composition of the electrolyte. In particular, the larger the area of the cathode in relation to that of the anode, the greater the rate of attack. Adverse area ratios are likely to occur with fasteners and at joints. Carbon steel bolts in stainless steel members should be avoided because the ratio of the area of the stainless steel to the carbon steel is large and the bolts will be subject to aggressive attack.
Stainless steels usually form the cathode in a bimetallic couple and therefore do not suffer corrosion. An exception is the couple with copper which should generally be avoided except under benign conditions. Contact between austenitic stainless steels and zinc or aluminium may result in some additional corrosion of the latter two metals. The corrosion is unlikely to be significant structurally, but the resulting white/grey powder may be deemed unsightly.
Bimetallic corrosion may be prevented by excluding water from the detail (e.g. by painting or taping over the assembled joint) or isolating the metals from each other (e.g. by painting the contact surfaces of the dissimilar metals). Isolation around bolted connections can be achieved by non-conductive plastic or rubber gaskets and nylon or teflon washers and bushes. This system is a time consuming detail to make on site and it is not usually possible to provide the necessary level of site inspection to check that all the washers and sleeves have been installed properly.
The development of stress corrosion cracking (SCC) requires the simultaneous presence of tensile stresses and specific environmental factors unlikely to be encountered in normal building atmospheres. The stresses do not need to be very high in relation to the proof stress of the material and may be due to loading and residual effects from manufacturing processes such as welding or bending. Caution should be exercised when austenitic stainless steel members containing high residual stresses, e.g. due to cold working, are used in chloride rich environments, e.g. swimming pools, marine, offshore and mainly if the temperature of the steel can reach 50°C or more, e.g. roofing.
The likelihood of SCC increases with increasing tensile stress and with increasing temperature. Ferritic and austeno ferritic stainless steels are in general completely proof against SCC. In austenitic stainless steels, an extra nickel content, a molydnenum addition reduces the sensitivity to SCC.
General corrosion is much less severe in stainless steel than in other metals. It only occurs when the stainless steel is at a pH value which is either very low (acid environments) or very high (alkaline environments) at high temperature. In normal building applications, general acid corrosion cannot occur. Sometimes it can occur in industrial or chemical atmospheres or most commonly by contact with products that are incompatible with stainless steels, e.g. hydrochloric acid used to "clean concrete or ceramics". Insurance of compatibility should be obtained from product information; reference should be made to tables in manufacturers' literature or the advice of a corrosion engineer should be sought.
When austenitic stainless steels are subject to prolonged heating between 450-850°C, the carbon in the steel diffuses to the grain boundaries and precipitates chromium carbide. This process removes chromium from the solid solution and leaves a lower chromium content adjacent to the grain boundaries. Steels in this condition are termed 'sensitised'. With a chromium level lower than 12% the grain boundaries become prone to general corrosion in pickling solution or to preferential attack on subsequent exposure to a corrosive environment. This phenomenon is known as weld decay when it occurs in the heat affected zone of a weldment.
There are three ways to avoid intergranular corrosion:
Experience has shown that a low carbon content (~0,05%) in most cases is sufficiently low to guard against intergranular corrosion after welding. This is particularly so when welding is done by arc processes (giving rapid heating and cooling), even for plate thicknesses up to 20mm.
The selection of the correct grade of stainless steel must take into account the environment of the application, the fabrication route, surface finish and the maintenance of the structure. Although the material has low maintenance requirements, where it is selected for use in a corrosive environment corrosion engineering needs to take a higher profile.
The first step is to characterise the service environment. The corrosiveness of an environment is governed by a number of variables such as humidity, air temperature, presence of chemicals and their concentration, oxygen content, etc. Moisture must be present for corrosion to occur. For example, heated and ventilated buildings can be classified as dry and corrosion is unlikely to occur in such environments. The risk of condensation is higher in areas such as kitchens and laundries. Coastal areas are very corrosive due to the presence of high concentrations of chloride ions in the air and structures exposed to sea spray are particularly prone to corrosive attack.
Having characterised the general environment, it is then necessary to consider the effect of the direct surroundings on the stainless steel (e.g. elements and substances which the material is likely to come into contact with). The surface condition, the temperature of the steel and the anticipated stress could also be important parameters.
Consideration should then be given to mechanical properties. The different types of loading should be defined, e.g. service loads, cyclic loads, vibrations, seismic loads. The effect of heating/cooling cycles may also need to be quantified. Ease of fabrication, availability of product forms, surface finish and cost also need to be considered.
Assessing the suitability of grades is best approached by referring to experience of stainless steels in similar applications and environments. Table 1, which is extracted from [2], gives guidance for selecting suitable grades for atmospheric environments. It is based on long term exposure of stainless steel sheet samples at a variety of locations. Expert advice should always be sought for more specialist applications, e.g. stainless steel immersed or in contact with chemicals.
Caution should be exercised when considering the use of "free-machining" stainless steels for fasteners. The addition of sulphur in the composition of these steels (commonly designated 303 in the austenitic class) renders them more liable to corrosion, especially in industrial and marine environments.
|
Table 1: Suggested grades for atmospheric applications |
||||||||||||
|
Steel grade |
Location |
|||||||||||
|
Rural |
Urban |
Industrial |
Marine |
|||||||||
|
L |
M |
H |
L |
M |
H |
L |
M |
H |
L |
M |
H |
|
|
430 |
/ |
(/) |
(/) |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
304, 302 (inc 304L, 321, 347) |
/ |
/ |
/ |
/ |
/ |
(/) |
(/) |
(/) |
X |
/ |
(/) |
X |
|
316 (inc 316L, 316Ti) |
O |
O |
O |
O |
/ |
/ |
/ |
/ |
(/) |
/ |
/ |
(/) |
|
L - Least corrosive conditions within that category, e.g. tempered by low humidity, low temperatures. M - Fairly typical of that category. H - Corrosion likely to be higher than typical for that category, e.g. increased by persistent high humidity, high ambient temperatures, particularly aggressive air pollutants. O - Potentially over-specified from a corrosion point of view. / - Probably the best choice for corrosion resistance and cost. X - Likely to suffer excessive corrosion. (/) - Worthy of consideration if precautions are taken (i.e. specifying a relatively smooth surface and if regular washing is carried out). |
||||||||||||
Note: Special ferritic or austenoferritic stainless steels and high alloys grade could be used in very corrosive environments. Consult manufacturers' literature.
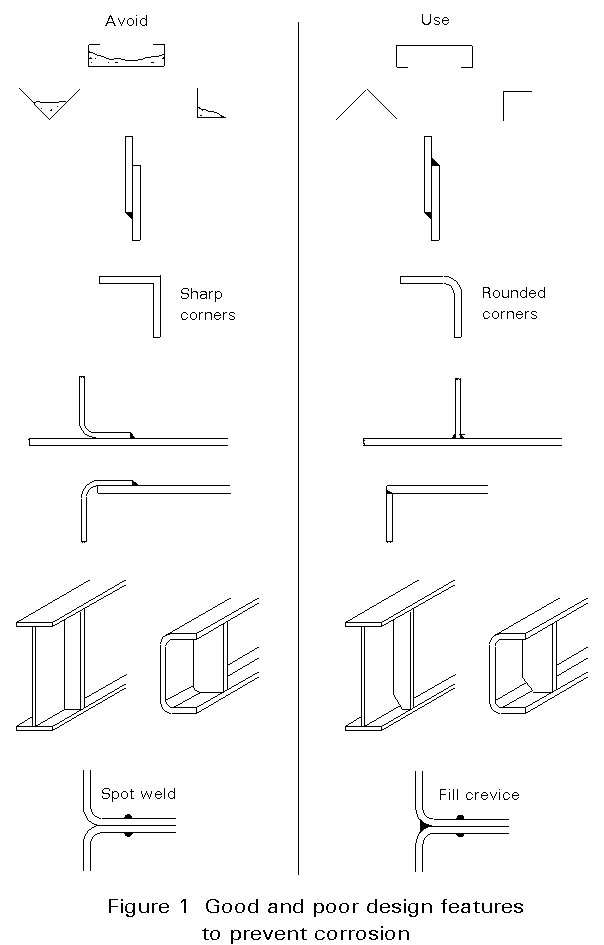
The main step in preventing corrosion problems is in selecting an appropriate grade of stainless steel with suitable fabrication procedures. As well as careful material grade selection, good detailing and workmanship can significantly reduce the likelihood of staining and corrosion whilst poor detailing and workmanship can be detrimental to corrosion performance. The following practical guidance of good practice will help ensure satisfactory performance of the material; not all points would necessarily be always applicable.
Figure 1 illustrates some good and poor design features to prevent corrosion.

All stainless steel should be carefully stored so that the surfaces are protected from mechanical damage and chemical pollution, e.g. ferrous contamination. Storage areas should be dry and clean. The use of protective films may be beneficial for architectural applications. Further advice can be obtained from the steelmaker.
It is necessary to avoid contamination of the surface of stainless steel components by carbon steel at all stages of fabrication, handling, storage, transportation and erection. In particular, the fabricator should take care to prevent contamination e.g. by quarantined work areas or specific maintenance procedures. This is to prevent carbon steel pick-up during rolling or from grinding debris or swarf which may rust when exposed to moisture and stain the surface. When carbon steel lifting or handling equipment such as strapping, crane hooks, chains or rollers are being used, suitable protective material should be placed between the stainless and carbon steel to prevent damage. Clean, heavy cardboard or light plywood are suitable materials for this purpose. Erection tools such as spanners and drifts should be stainless steel to ensure surface contamination does not take place. Grinders should also be reserved exclusively for use on stainless steel.
Contact with organic contaminants such as oils, greases, dyes, glues, adhesive tape and other similar deposits should be avoided. When they are used, their suitability should be checked with their manufacturer. Stainless steel can be disfigured by certain chemicals and checks should be made to ensure that any erection marks penned on the surface can be easily removed.
The designer must specify any visual requirements so that the fabricator can take due care to protect the particular surface.
Should components require cleaning for aesthetic reasons, soap, detergent or a solution of ammonia may be used with scrubbing brushes. The stainless steel should be rinsed down afterwards with clean water and then wiped dry. Inspections to detect signs of mechanical damage, surface contamination or incipient corrosion attack are recommended for exposed architectural features.
Strong acid solutions are sometimes used to clean the masonry and tiling of buildings but they should never be permitted to come into contact with any metal, including stainless steel., If this should happen, the acid solution must be washed off immediately with generous amounts of water.
[1] Burgan, B. A., Concise guide to the structural design of stainless steel, The Steel Construction Institute, SCI-P-123, Second Edition, 1993.
[2] Nickel Development Institute, An architect's guide on corrosion resistance, NiDI, 1990.