ESDEP WG 18
STAINLESS STEEL
To provide an overview of the basic aspects of the stainless steels. To give practical information as an introduction to the succeeding lectures.
Lecture 18.2: Structural Behaviour and Design
Lecture 18.3: Corrosion of Stainless Steel
Lecture 18.4: Fabrication
A brief history is given of the development of stainless steels. Background information on composition, stainless properties and differences from carbon steel is provided.
A general overview of the stainless steels by grouping them into families in accordance with their metallurgical structure is introduced.
The lecture continues by giving practical information on designations of alloys in the various systems used, bolts and nuts, distinguishing the various stainless steels, reasons for their use, product forms available, and surface finishes.
Typical applications are given to illustrate the use of stainless steel in steel construction.
Stainless steels are modern materials. Ever since they became available to industry their use has constantly extended into new applications. This process continues even today.
To make successful use of the stainless steels in building applications, it is necessary to know their properties, their capabilities regarding corrosion resistance, the availability of product forms and surface finishes. It is also of interest to be aware of reference applications that prove the success of the selection of stainless steels many years ago.
This lecture is designed to provide an overview of the stainless steels, their properties and available product forms, which, together with the succeeding lectures, will enable successful use of them to be made in designs that will be cost efficient in terms of corrosion resistance, maintenance and durability. Stainless steels are friendly to the environment; they actively and passively help to keep it clean and they are recycled to a high degree (50 - 70 %).
The making of stainless steel is a technological art requiring skill and special equipment in order to keep critical elements within specified limits. Stainless steels contain substantial amounts of expensive elements such as chromium, nickel, molybdenum, etc. justifying their higher cost of approximately five times that of carbon steel. However, the material costs are only part of the total cost of a structure. A good design will take advantage of the properties of the stainless steels and result in a cost effective solution which can be demonstrated by life cycle cost calculations.
An up-to-date design approach takes into consideration the effects of maintenance, periodic repairs, replacement and shut downs in addition to environmental aspects.
A stainless steel structure designed on the basis of a carbon steel design results almost certainly in an unsatisfactory solution.
It has been known for nearly 200 years that modest amounts of chromium alloyed to common steel improves its corrosion resistance in air and water and that increasing amounts of chromium increased this resistance. These investigations were made in 1821 by Berthier in France. This knowledge however could not be used in steel making because of limitations in technology. It was impossible at that time to reduce the high carbon content of melts to sufficiently low levels, or to control the chromium content. The chromium content was always either too low or too high. High carbon and/or chromium content resulted in brittle alloys, a low chromium content in insufficient corrosion resistance.
After the turn of the century numerous researchers worked on these alloys and published papers. Goldschmidt in Germany found a method of producing ferro-chromium with a very low carbon content. On the basis of this discovery A. Portevien and L. Guillet in France and W. Giesen in Germany published papers with the results of their research on martensitic 13% and on ferritic 17% chromium steels between 1904 and 1909. L. Guillet presented a study on chromium-nickel steels with an austenitic structure in 1909.
These alloys were already similar to today's alloys and the three major metallurgical families of stainless steels, namely the martensitic (hardenable by heat treatment), ferritic (non-hardenable) and the austenitic (non-hardenable) steels. At the same time P. Monnartz in Germany defined the role of passivity in corrosion resistance. These researches took place in laboratories and from 1910 - 1915 attempts were made to develop larger-scale practical production for the stainless steels. This work involved melting the alloys in greater quantities, converting the ingots into semi-finished forms, and fabricating them into equipment. Although there were many researchers involved in the development of the first stainless steels, credit is usually given to the following metallurgists for having made major advances and contributions to the art:
Ordinary steel is composed of iron (Fe) with certain other elements which result from steelmaking such as carbon (C), manganese (Mn), silicon (Si), phosphorus (P) and sulphur (S). A typical unalloyed carbon steel used in construction has the following chemical analysis (in % of weight):
|
C |
Mn |
Si |
P |
S |
Fe |
|
0,17 |
0,60 |
0,25 |
0,045 |
0,045 |
> 98 |
If a minimum of 11% of chromium is added to such a steel a "stainless steel" is obtained. The chemical analysis (again in % of weight) thus becomes:
|
C |
Mn |
Si |
P |
S |
Cr |
Fe |
|
0.10 |
1,0 |
1,0 |
0,045 |
0,030 |
11 |
£ 87 |
Iron is still the dominant element but the addition of chromium requires also minor changes in the content of manganese and silicon to facilitate steel making, and of phosphorus and sulphur. These latter two elements are impurities and have a detrimental influence on a number of properties of the steel, such as the corrosion resistance and the weldability. A steel with 11% of chromium represents the simplest form of a stainless steel. It has sufficient corrosion resistance to resist a mild aqueous environment.
The addition of chromium to a steel results in the formation of a thin, tight, adherent and ductile layer of primarily chromium oxide on the surface of the steel provided that it is exposed to air or another oxidizing environment. Since this layer conveys passivity to the steel, which means that it does not actively corrode, it is also called a passive layer. It is responsible for the ability of the steel to resist corrosion. The thickness of this very thin layer is of the order of 1 - 10nm (1 nanometre = 10-9 m or 0,000001 mm). The passive layer is not inert or of a given, unchanging thickness or composition, but depends on the composition of the steel, the treatment given to the surface and the corrosive influence of the environment it is exposed to. If changes to these conditions occur the passive layer adapts itself.
It is also possible that the passive layer is damaged by tools during manufacturing (milling, grinding, polishing, drilling, tapping) or by accident. Under normal conditions (in the presence of air) the passive layer forms itself anew; it is self-healing. This interesting capability of stainless steel is of great practical importance as no special measures are needed to renew or repair the corrosion resisting layer.
Coatings, which are applied for the protection against corrosion of other materials, do not, because of their nature, cover the surfaces entirely and are prone to damage. Damage to coating is usually the starting point of corrosion.
The family of the stainless steels comprises a great number of different alloys. They were each developed to meet specific needs such as higher corrosion resistance, improved mechanical properties, such as higher strength, hardness or ductility, metallurgical stability under the influence of welding heat, and in special cases improved machinability. Since all these steels contain at least 11% of chromium, they are all protected by the passive layer forming spontaneously on the surface.
The designation of "rustless" or "stainless" steel goes back to the early years of metallurgical development. Its meaning was that these novel alloys of steel would not rust or stain when exposed to the atmosphere or to water. This designation is still very much in use today but it can be misleading to the uninformed designer. A much wider interpretation that "stainless steels" are resistant to every conceivable corrosive environment is not correct. Keeping this limitation in mind it is nevertheless a very practical designation for the all encompassing description of the entire family of these steels.
There are a number of important reasons why stainless steels are used extensively in structures. They are:
Atmospheric Corrosion Resistance and Durability
Austenitic stainless steels have a long history of successful applications in the building industry. Their excellent corrosion resistance is the prime reason for low maintenance costs and excellent durability. These properties are becoming increasingly important in any building project.
Aesthetics
The surface of stainless steels can be obtained in many different qualities such as mirror polished, ground with different grit sizes, brushed, cold rolled, sand blasted, roll textured and coloured. Stainless steel combines aesthetically with any other material without dominating it yet maintaining its timeless elegance especially if slender (but strong) elements are used.
Hygienic Aspects and Cleanliness
Stainless steel has a hard, smooth and uniform surface that lends itself for demanding applications where hygiene and cleanliness are important. For these reasons it finds many uses in hospitals, laboratories, baths and swimming pools.
Heat Resistance
The stainless steels are heat resisting materials. They outperform any other conventional structural material in fire or high temperature applications. Their use is indicated for fire escape systems, fire doors, enclosures, cable trays, chimneys, etc. (See also Section 5.1).
There are a number of advantageous properties, which can be utilised in certain applications:
Strength
Stainless steels have very interesting mechanical properties that can be varied within wide limits due to their response to cold work. Designs that make good use of the advantageous mechanical properties are cost efficient.
High Energy Absorption
The austenitic stainless steels are tough and ductile resulting in an exceptionally high plastic deformation before they fail. These properties may be important in safety barriers, blast walls and for aseismic building frames.
Ease of Fabrication
The stainless steels can be fabricated just like conventional metallic materials. They can be sheared, cut, sawn, bent, drawn, roll formed, drilled, milled, welded, extruded, ground and polished.
Favourable Life Cycle Costs
It has often been shown that the use of stainless steels results in favourable life cycle costs which take into account all connected costs over the expected life of an application. Life cycle cost calculations are becoming increasingly important in view of the high costs of maintenance, shut-downs, demolition and replacement of equipment and parts. Life cycle cost calculations include such items as initial installation costs, maintenance costs, the cost of shut-downs, repair and replacement costs, interest rates and the effect of inflation.
Recycling and Protection of the Environment
It is not well known that the recycling of stainless steel is already developed to a high degree. Depending on economic conditions the recycling amounts to between 50% and 80%, a percentage not achieved with most other materials. One reason for this favourable aspect is the fact that stainless steel scrap is a valuable commodity which can be sold at any time.
Recycling involves storage, transportation and handling in different locations. Stainless steel, because of its corrosion resistance, has absolutely no effect on the environment even if it is left exposed to the weather for years.
Stainless steel plays also an important active part in the protection of the environment. It is used extensively in vehicle exhaust systems and catalytic convertors, sewage treatment plants, chimneys, smoke scrubbing and other such applications.
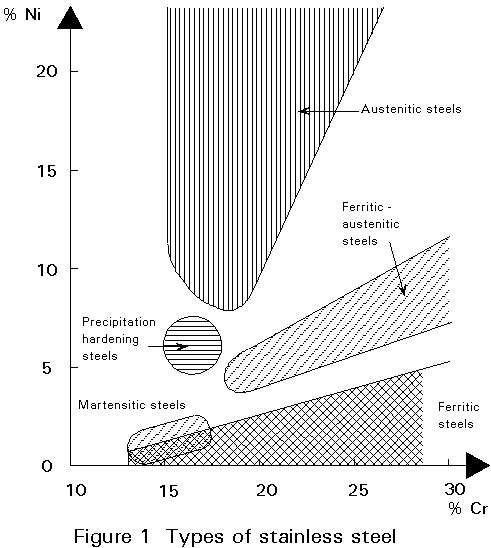
It is practical to group the numerous different stainless steels in accordance with their metallurgical structure. On this basis the following groups can be distinguished:
Figure 1 provides a graphical overview of these groups with respect to chromium and nickel content.

In structural applications, the austenitic grades are used predominately.
The favourable corrosion resistance of austenitic stainless steels due to their chromium content is combined with favourable mechanical properties (good ductility and toughness) and good weldability due to a certain nickel content (see Figure 1).
Austenitic stainless steels contain chromium (17 - 20%) nickel (8 - 17%), and they have a low carbon content (usually less than 0,10%).
They can contain other elements such as molybdenum (2 - 6%), titanium or niobium in order to stabilize the structure and sulphur which is added to improve machinability.
The chemical analysis of a typical austenitic stainless steel is:
C £ 0,10%
Cr = 16,5 - 18,5%
Ni = 10,5 - 13,5%
Mo = 2,0 - 2,5%
Ti ³ 5 x % C
Austenitic stainless steels in the most corrosion resisting state (solution annealed) are non-magnetic. For higher strength they can be cold worked by rolling, bending, pressing, etc. which can make them slightly magnetic. They are readily weldable.
Typical applications are in architecture, roofing, fasteners, food processing, chemical and pharmaceutical industries, hospitals, medical uses, transportation, household, chimneys, paper industry, nuclear installations, watch casings and straps, etc.
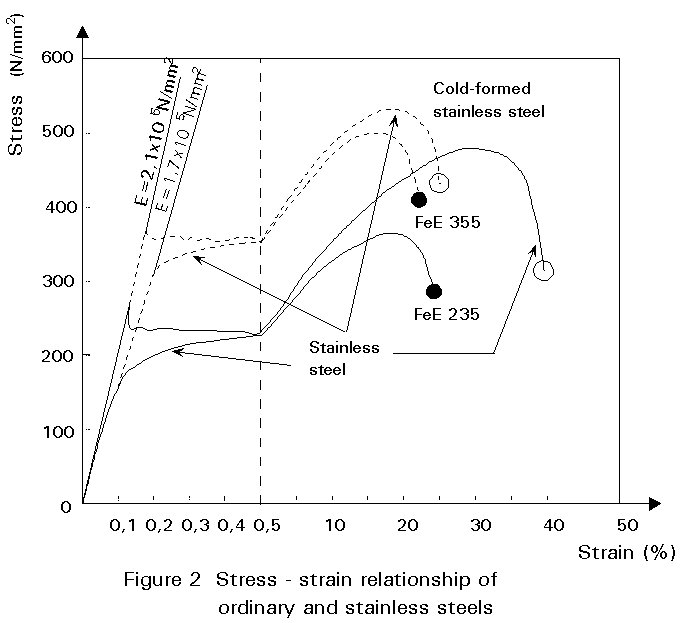
The mechanical behaviour of austenitic stainless steel is highly influenced by the cold working process. Data for typical steels are given in Table 1 and in Figure 2 by the stress-strain diagram. It shows also the significant difference of the Young's modulus.
There are a number of stainless steels that according to their specific properties are used in special applications. In certain cases they may also be used in building structures. They are:
Martensitic Stainless Steels
These steel contain from 12% to 18% chromium and from 0,12% to 0,9% carbon. Due to the presence of rather high carbon contents they can be hardened. Some of these steels contain modest amounts of nickel (up to 2,5%) and molybdenum (up to 0,6%) in order to improve their corrosion resistance. Other elements added can be titanium, vanadium and niobium. A high carbon content reduces the corrosion resistance.
Martensitic stainless steels are magnetic and they are not welded as a rule.
Typical applications are in mechanical engineering for pump shafts, valves, dies, turbine blades and roller bearings. Another area of application is for household and industrial knife blades.
Ferritic Stainless Steels
Ferritic stainless steels contain from 12% to 18% chromium just like the martensitic stainless steels but their carbon content is usually less than 0,08% although sometimes it may be up to 0,15%. There are special ferritic steels with a chromium content of up to 29%.
These steels can contain other elements such as molybdenum (up to 1,2%), titanium or niobium (both these elements are used to stabilize the structure) and sulphur which is added to improve machinability. For higher strength, nitrogen is added in small amounts, from 0,10 - 0,30%.
Ferritic steels are magnetic. With proper precautions they can be welded.
Typical applications are vehicle exhaust systems, containers, hot water tanks, dish washers, washing machines, kitchen tools.
Austenitic/Ferritic Stainless Steels (Duplex Steels)
Duplex stainless steels have a high chromium content of 20 - 25%, a low nickel content of 5 - 9%, a varying molybdenum content of 0,2 - 3% and a low carbon content of less than 0,06%. They contain nitrogen in amounts of 0,07 - 0,20% to increase strength and to stabilize the duplex structure and sometimes copper of up to 2,5%.
Duplex stainless steels are magnetic and can be welded with proper precautions.
Typical applications are in the paper, chemical, oil and building industries.
Precipitation Hardening Stainless Steels
Solution annealed austenitic stainless steels have a very modest 0,2% yield strength. On the other hand their corrosion resistance and ductility (toughness) are at a maximum. Over the last 50 years many attempts have been made to combine high mechanical strength with good corrosion resistance. The compromise between these opposing properties resulted in a number of precipitation hardening stainless steels. However they require extensive experience from both the steel producer and the user to select the appropriate alloy for a given application.
The high mechanical properties are obtained by a closely controlled heat treatment which results in increased hardness, higher yield and ultimate tensile strength and improved fatigue resistance.
The martensitic and semi-austenitic precipitation hardening stainless steels are magnetic. The austenitic steels are not magnetic.
The precipitation hardening stainless steels can be welded provided suitable procedures are followed. Alloys with high phosphorus content are difficult to weld.
Typical applications are in aeronautical and space applications, parts for turbines, motors and compressors, corrosion resisting springs, missiles, pressure vessels and highly stressed parts in research facilities.
There are several alternatives for designating stainless steels. They are mentioned here because they are often found in technical descriptions.
This system makes use of the elements added to the steels. It becomes cumbersome with the more highly alloyed steels as the designations become long. The elements are referred to in descending order of contained weight. Thus we have:
In the case of a low carbon steel or a steel stabilized with titanium one would say:
The advantage of this system is that even a not-so-well informed reader understands readily what kind of steel is referred to. For a more exact description it would be necessary to add the content of each element in % of weight.
The system introduced by the American Iron and Steel Institute (AISI) is in general use world-wide. It defines each grade of steel with a number and if needed with an additional letter. Examples are as follows:
L = Low carbon
N = Nitrogen
Se = Selenium
Ti = Titanium
C = High carbon
B = Lower carbon than C
A = Lower carbon than B
F = Free machining (high sulphur content)
Cb = Columbium = Niobium Nb
Thus 304LN stainless steel means that it is an austenitic steel with low carbon content (0,03% maximum) and nitrogen. Nominal chromium and nickel contents are 18 - 20% and 8 - 12% respectively.
The 200 and 300 series are reserved for austenitic steels, the 400 series for martensitic and ferritic steels and the 600 series for precipitation hardened steels.
For the exact chemical composition and properties of a steel, it is necessary to refer to the standard. Some typical designations are:
Type 201: C = 0,15% maximum
Mn = 5,5 - 7,5%
Cr = 16 - 18%
Ni = 3,5 - 5,5%
Type 304: C = 0,08% maximum
Cr = 18 - 20%
Ni = 8 - 10,5%
This German system is widely used in other counties as well. Each designation consists of 5 numbers and for details of composition and properties of the steels one has to refer to the standard. As an example, material number 1.4306 will be considered:
The first digit is 1 and indicates that it is a steel.
The two following digits "43" signify "chemically resisting steels without molybdenum, columbium or titanium".
The last two digits "06" define the exact alloy.
In addition to the designation "43" there are also the following ones:
"40" without molybdenum, columbium or titanium, nickel less than 2,5%
"41" with molybdenum, without columbium or titanium, nickel less than 2,5%
"44" with molybdenum, without columbium or titanium, nickel more than 2,5%
"45" with copper, columbium or titanium, nickel more than 2,5%
A steel to material number 1.4306 has the following composition:
C = 0,03% maximum
Cr = 18 - 20%
Ni = 10 - 12,5%
It corresponds therefore to AISI type 304L stainless steel although the lower limit of nickel is higher by 2%.
This system is widely used by a number of standards. It consists of a series of letters and numbers as in the following examples:
X 2 Cr Ni 18 11
X means that it is a highly alloyed steel
Cr stands for chromium and 18 is the content in %
Ni stands for nickel and 11 provides an indication of the content in %.
A steel to X 2 Cr Ni 18 11 corresponds to AISI type 304L and to material number 1.4306.
National standards at present make use of these systems as follows:
AISI-System: - British Standards (BSI)
- Japanese Standards (JIS)
Abbreviated System: - Italian Standards (UNI)
- German Standards (DIN)
- Spanish Standards (UNE)
- EURONORM
- ISO/DR 2604/4
- The French Standards (AFNOR) use different letters but similar numbers (X 2 Cr Ni 18 11 = Z 2 CN 18 10)
Numbering System: - German Material Numbers
- Swedish Standards (SIS)
- ISO/DR 683/13 and DR 2604/1-75
(Swedish and ISO standards numbering systems are not explained in this lecture)
In view of this rather confusing situation it is hoped that in the near future only 2 systems will prevail:
For special applications many of the stainless steel alloys are available in the cast form or as forgings. Smaller parts can be produced by powder metallurgy but by far the greatest use is made of wrought product forms that are available as follows:
The strength of austenitic stainless steels cannot be improved by heat treatment. Higher strength levels can be easily obtained by cold working, e.g. cold rolling, roll forming and press brake bending. Typically, 15% cold working doubles the 0,2% yield strength. It is possible to order material to the strength levels required. Cold worked material may have a slightly reduced corrosion resistance.
For details refer to Lecture 18.4, Fabrication.
The austenitic stainless steels can be welded with all known processes but for the other metallurgical groups restrictions apply. Whatever the grade of stainless steel which has to be welded it is necessary to select the most cost effective procedure.
Stainless steels for structural applications are selected primarily for their corrosion resisting properties. It must be assured that the weld matches the properties of the base material.
Austenitic steels can be welded to structural carbon steel by observing proper precautions.
For details refer to Lecture 18.4, Fabrication.
Numerous finishes are available for surfaces of stainless steel parts providing the architect/engineer with a wide range of effects. Cold rolled sheet, strip, tubing and bars can be obtained with the following finishes:
In addition sheet is obtainable in a great number of patterned and textured surfaces produced by rolling.
Stainless steel surfaces can be coloured by a special process.
Screws, bolts and nuts made of various grades of stainless steel are grouped by ISO Standard 3506 on the basis of the following three metallurgical structures:
There are steel groups, which are identified with abbreviations as follows:
For structural application only A2 and A4 should be used, since the rest of the groups have a reduced corrosion resistance.
Table 2 summarises the designation of fastening elements.
Bolts to A2 and A4 can be obtained in 3 grades of ultimate tensile strength:
Stocks of suppliers are based on this strength class and the available selection is greatest.
Table 3 provides an overview of the mechanical properties of these bolts.
Nuts without indication of a strength class correspond to class "80", e.g. the highest class. The strength classes of "70" and "50" are only marked on them if the strength test indicates that they did not correspond to the highest class.
Without exception bolts should have rolled threads since cut or milled threads have a higher tendency to seize which can result in lower strength of a connection. Screws of stainless steel should always be assembled with a suitable lubricant as the frictional resistance between two parts made of stainless steel is significantly higher as compared with carbon steel bolts.
Bolts designation in accordance with ISO 3506 (hexagonal head and hollow head cap screws) have to be marked from size M5 and larger according to Table 4.
Table 5 gives a list of structural applications of stainless steels. Some examples are given below of important applications using stainless steel products.
Erected 1926 - 1929
The entire spire down to the first platform is covered in AISI type 304 stainless steel. It was cleaned in 1961 and found to be in perfect condition despite the aggressive New York atmosphere and closeness to the ocean.
Erected 1990 - 1991
The church roof, a reinforced concrete membrane, is in part supported by two hanger rods of 70 mm diameter and 18 m length. The material selected was AISI type 316LN stainless steel.
Architect: Guglielmo Giani
Structural engineers: Antonio Migliacci and Danilo Campagna
Attachment of a hanger rod to the concrete church roof described under 2 above.
Outside: AISI type 304 or 316
Inside: AISI type 321, 316 or 317
Erected 1977
The roof with a total weight of 870 tonnes is carried by a spider like structure with 6 legs designed as trusses made of tubes. The material chosen was AISI type 316.
Architect: Wilfred Beck-Erlang
Structural engineer: Ing. Büro Pieckert
Photos: Beck-Erlang, Baacke.
Detail of one of the 42 suspension points for the planetarium roof of the structure described under 5 above.
Non-slip floor grating made of stainless steel AISI type 304 or 316 as used in the chemical, pharmaceutical, food processing, beverage, and plating industries.
720,000 prefabricated concrete segments for the tunnel lining were equipped with AISI type 304 stainless steel attachments for transportation, handling and positioning the segments inside the tunnel.
Advertising Tower of 22m height at Calbusera in an industrial area of Milan, Italy. The tower is covered with 500m2 of dark blue coloured stainless steel sheets to AISI type 316.
Design: Giovanni Baroni, Gerardo Genghini and Massimo Pellacini
|
Material |
|
Minimum 0,2% yield strength [N/mm2] |
|
|
*1.4301 1.4541 1.4401 1.4571 |
** X 5CrNi 18 10 X 6 CrNiTi 18 10 X 5 CrNiMo 17 12 2 X 6 CrNiMo 17 12 2 |
Solution annealed |
195 200 205 210 |
|
1.4301 K 700 |
X 5 CrNi 18 10 K 700 |
Cold formed to strength level indicated
|
350 |
|
1.4541 K 700 |
X 6 CrNiTi 18 10 K 700 |
350 |
|
|
1.4401 K 700 |
X 5 CrNiMo 17 12 2 K 700 |
350 |
|
|
1.4571 K 700 |
X 6 XrNiMo 17 12 2 K 700 |
350 |
|
* Material Number System
** Abbreviated System of Destination
Table 1 The Material Properties of Austenitic Stainless Steel, DIN
Division into 3 metallurgical groups:
- austenitic (A)
- ferritic (F)
- martensitic (C)
|
Steel Group |
Austenitic |
Ferritic |
Martensitic |
|||||||||||||||
|
Identification of the Steel Groups |
|
|_________ |
|
| |
|
|
__ |
|__ |
___ |
|
|
|||||||
| ____ |
|__ |
| |
|
|
| |
|
___ |
|_ |
|
| |
||||||||
|
|
| |
|
| |
|
| |
|
|
|
| |
|
|
| |
|
| |
|
| |
||
|
A1 |
A2 |
A4 |
|
|
F1 |
|
C1 |
C4 |
C3 |
|||||||||
|
|
|_____ |
|_____ |
| |
|
|
|
| |
|
|
|__ |
__ |
| |
|
| |
||||
|
|
|
______ |
| |_____ |
|
|
______ |
| |___ |
___ |
| |__ |
| | |
|
|||||||
|
|
|
| |
|
| |
|
| |
|
| |
|
|
| |
| |
|
| |
|
| |
||
|
Strength Classes |
|
50 | |
70 | |
80 | |
45 | |
60 | |
50 | |
70 | |
80 | |
|||||||||
|
|
|
| |
|
| |
|
| |
|
| |
|
|
| |
| |
|
| |
|
| |
|
|
|
|
soft |
cold worked |
heavily cold worked |
soft |
cold worked |
soft |
heat treated |
heat treated |
||||||||||
Table 2 Designation of Fastening Elements made of Corrosion Resisting Steels to ISO 3506
|
Material grade
|
Grade
|
Property class
|
Range of diameter
|
Bolts |
|
|
Stress at 0,2% permanent strain1) fyb (N/mm2) |
Tensile strength1) fu (N/mm2) |
||||
|
Austenitic
|
A1, A2, A4 |
50 |
£ M 39 |
210 |
500 |
|
A2, A4 |
70 |
£ M 202)> M 20 to £ M 30 |
450 250 |
700 500 |
|
|
A2, A4 |
80 |
£ M 202) |
600 |
800 |
|
|
Note: Specified values for minima. 1) All values are calculated and related to the stress cross-section of the thread.2) For property classes 70 and 80, values must be agreed with the manufacturer for lengths greater than 8 diameters or for sizes greater than M30 and M20 respectively. |
|||||
Table 3 Mechanical Properties of Fasteners made of Austenitic Stainless Steels (ISO 3506)
Manufacturer's Identification
|
|
A |
2 |
- |
70 |
||||
|
Short designation of material: |
|
|
|
|
|
|
|
|
|
A = Austenitic chromium-nickel (molybdenum) steel |
|
|
|
|
|
|||
|
Short designation of steel group: |
|
|
|
|
|
|
|
|
|
1 = Free machining steel with sulphur 2 = Chromium-nickel steel 4 = Chromium-nickel-molybdenum steel |
|
|
|
|
||||
|
Short designation of strength group: |
|
|
|
|
|
|
|
|
|
50 = 500 N/mm2 70 = 700 N/mm2 80 = 800 N/mm2 |
||||||||
Table 4 Marking of Screws and Nuts
|
GENERIC APPLICATION |
SPECIFIC APPLICATION |
PRODUCT FORMS |
|
Lattice structures |
× foot bridges× roof trusses× space-frames (roofs)× power transmission masts |
Cold formed sections, tubulars and hollow sections |
|
Cladded structures |
× roof and wall cladding× stressed skins× control rooms× freight containers× lorry/coach bodies |
Sheet and hot rolled sections |
|
Columnar structures |
× high mast lighting columns× telephone poles× chimneys× street furniture |
Cold formed sheet or plate, tubulars |
|
General structures |
× staircases/stairtowers× walkways× handrailing/balustrades× balconies× overhead gantries× racking× turnstiles× bus shelters× swimming pool accessories× external lift guides× silos/bunkers/chutes/hoppers× air conditioning components× paint shop crane rails |
Cold formed sections, plate, tubulars and hollow sections |
|
Building frames |
× seismic resistant frames |
Hot rolled sections |
|
Preformed components |
× blast walls× floor planks× cable trays× ventilation louvres× glazing bars× heat shields× building accessories (shelf angles, lintels, reinforcement bar, fixings etc). |
Sheet, plate, bar and extrusions |
Table 5 List of structural applications