ESDEP WG 4A
PROTECTION: CORROSION
This lecture is intended to give detailed information on the corrosion protection of steel components in bridges for the design engineer.
Lecture 4A.2: Factors Governing Protection of Steelwork
Bridges are normally built for a long service life. They are subject to an unfriendly environment. For economic reasons a high quality corrosion protection is required. For the main structure painting or weathering steel is the normal choice and, for smaller details, hot dip galvanising or stainless steel is also used. The possibility of unpainted steel in closed sections is discussed.
Bridges are generally built for a long service life, e.g. 100 years, and they are subjected to attack arising from their use and from the environment: loads, wind, accidental damage, rain, deicing salts, sun, etc.
To ensure a sufficient life, a correct degree of protection against corrosion is required.
There are numerous means for preventing corrosion of steel structures, but for bridges the usual method is to use coating systems - paint or metallic coatings - or, for some applications, alloyed steels - weathering and stainless steels.
Several parameters influence the choice of a protective method: the required lifetime, the environment, design and economic considerations.
The required lifetime of the protection system is not the same as the lifetime of the steel structure it protects. The protective system usually required has a life in the order of 7 to 30 years between two periods of maintenance, depending on the severity of environment and on the age of the structure (7 to 15 for old bridges - 10 to 30 for new ones).
The environment influences the type and the intensity of the corrosion.
The influences of macro- and micro-climate should be considered:
a. macro-climate is the general environment in which the structure is situated. Macro-climates can be roughly divided into:
but in practice, the actual environment is often a combination of these categories. The urban environment, for example, may be a semi-polluted inland macro-climate.
b. micro-climate is the direct environment of the bridge elements and is affected by the configuration of the structure:
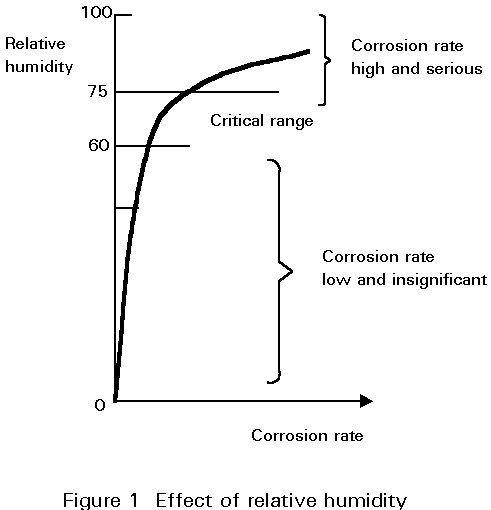
Atmospheric corrosion occurs only if the air is sufficiently wet and polluted.
Under a critical level of relative humidity of 60%, the corrosion rate of steel is very low and insignificant.
Above 75% relative humidity, corrosion rates begin to be serious (see Figure 1). For a bridge this level of humidity is exceeded for long periods.

Presence of chemical pollutants in the atmosphere can increase its corrosivity:
The different parts of bridges are not subjected to the same conditions of exposure see, for example, Figure 2. Corrosion is more or less severe depending on the conditions at locations which are:
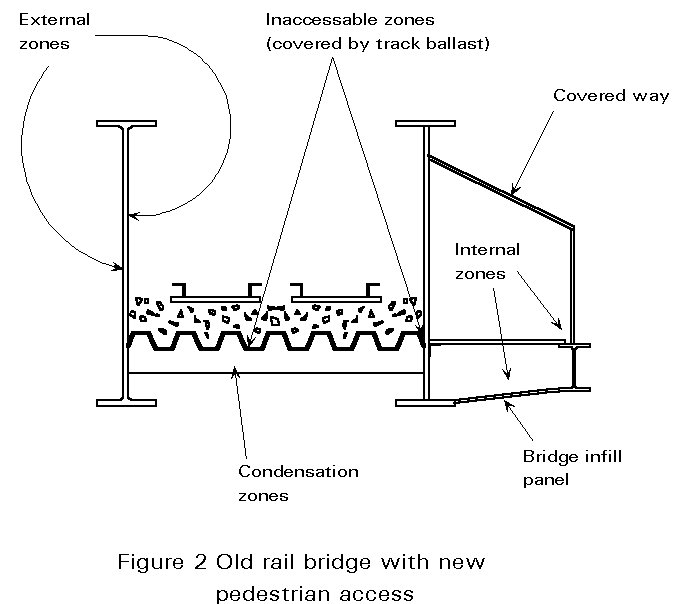
External locations are directly exposed, alternately wet and dry, and subject to the influence of temperature changes and UV radiations.
Condensation zones have permanent humidity, a high concentration of pollutants, possibility of localised corrosion.
Areas not washed by rain and permanent humidity.
Internal locations have no corrosion development if there is no renewal of the air, or localised corrosion if condensation occurs due to wet air (inside of girders).
General information on surface preparation and coatings is given in Lecture 4A.2.
Depending on environment, type of element, expected lifetime and facilities for maintenance, the choice of a protective system is made from the following:
Zinc has been used as a protective coating for steel for many years. It is by far the most widely used metal for protecting structural steel against corrosion. Zinc has a good behaviour in normal inland and coastal environments, but is quickly corroded in acid atmospheres such as may occur in industrial or polluted urban environments. In this case, aluminium or an aluminium-zinc alloy is a more suitable protection.
Brief details of these coating are:
It is described in Lecture 4A.2. A thickness between 60 and 100 microns is needed normally for anticorrosion effectiveness of 10 to 12 years.
Usual thickness:
- zinc or Zn/Al 85/15 120 microns
- aluminium 150 microns
Effectiveness against corrosion can last for 15 years if associated with paint coatings.
In some cases where it is necessary to provide an extra protection of the zinc coating a paint-coating should be applied. Paint coatings on zinc surfaces have to meet special requirements and paint manufacturers should be consulted.
Stainless steels are well known for their resistance to corrosion in corrosive atmospheres. This resistance is due to the presence of alloying elements, such as chromium and nickel, which provide a passive protective layer on the surface.
An example is the austenitic type with 17-18% Cr and 7-9% Ni. This alloy is resistant to corrosion only as long as the passive layer is not removed. In contact with slightly acid water the passive layer dissolves and the steel starts corroding. A more resistant alloy is achieved by adding molybdenum, e.g. the austenitic type with 17% Cr, 11%Ni and 2-3% Mo. This alloy is suitable for coastal and industrial environments (specially good resistance against chlorides).
Precautions must be taken when using stainless steel to avoid galvanic corrosion when in contact with carbon steel, zinc or aluminium.
In bridge construction, stainless steel can be used for secondary elements, connections, and ancillary equipments.
Weathering steel (see Lecture 4A.2) has been used for bridges for more than 30years with quite mixed results. The experience shows that weathering steel can be used successfully under certain conditions. A survey in the USA has clearly pointed out the most important reason for lack of success, i.e. the use of de-icing agents. States in the north that use de-icing agents have experienced heavy corrosion on bridge girders of weathering steel. This record should be considered together with the fact that North American bridges rarely have waterproofing of the bridge deck and the joints may not be watertight. Although the bridge deck may be made watertight initially, there is always a risk of leaks in the future. The combination of weathering steel and de-icing agents should therefore be avoided.
For the development of the protective patina it is essential that weathering steel occasionally gets wet and then dries. Thus in design nominally horizontal surfaces should be avoided because they may trap water due to imperfections. For instance a horizontal bottom flange should preferably be formed like an inverted V.
Even if no de-icing agents are used, water leaking through the bridge deck or joints is harmful in large amounts. Care should be taken to make the deck as watertight as possible.
A steel tube that is completely sealed will not corrode inside. This features is useful for small sections that may be closed effectively. However, a box girder bridge with a concrete deck cannot be considered to be completely closed. The concrete cracks and water may enter as well as oxygen. For this reason box girders are normally painted on the inside but with a less comprehensive paint system.
Another possibility is to close the box as far as possible and to install dehumidifiers that keep the air at less than 60% relatively humidity. The equipment and its running costs are inexpensive.
The danger of corrosion can be reduced substantially by careful attention to design.
A design that takes into account all possible ways of preventing corrosion is much better than one where the fight against corrosion relies only on the protection of the steel surface.
Anti-corrosion actions should ideally be considered at the planning stage and at the latest on the drawing board.
Some discussion of maintenance is included in Lecture 4A.2.