ESDEP WG 4A
PROTECTION: CORROSION
To give young architects and engineers a basic understanding of the corrosion process and the practical means of protecting structural steelwork.
None
This lecture presents the theory of corrosion in a very simple way. The galvanic series and galvanic corrosion are covered briefly. The question of why structural steel requires protection against corrosion is discussed and the fundamental considerations relating to protection are described, e.g. establishing the environment, the choices of protective coatings, surface preparation options and other considerations, e.g. chemical cleanliness.
The more common metals exist in nature as metallic compounds. The principal compounds or ores are oxides and sulphides.
The extraction process is:
Compound ® Metal
Metals spontaneously react with any liquid or gaseous environment in which they are placed and a corrosion product is produced which is very similar to the original ore from which the metal was obtained. Thus:
Iron Ore = Iron Oxide
Rust = Iron Oxide plus chemically bonded water.
Corrosion processes are chemical reactions taking place at the surface of the metal. They obey well established chemical laws - which is fine if you know them! Most of us do not need to know them because we are not dealing with corrosion problems daily. The purpose of this lecture is to describe the main types of corrosion met in ordinary buildings, structures, plant, factories, etc.
Corrosion products may act as a barrier between the metal and its surroundings, effectively slowing down the corrosion rate. This phenomenon is frequently observed when metals corrode in air, a process known as "dry corrosion". It cannot be expected to happen when the corrosion products are soluble and the corrosion is taking place in an aqueous environment, i.e. "wet corrosion". For example, in a dry environment
Zinc + Oxygen ® Zinc Oxide + Water + Oxygen
Nothing much happens!
But add acid condensate (as frequently occurs in industrial environments) and,
Zinc Oxide + Sulphuric Acid ® Zinc Sulphate + Water ® Eases away, exposing Zinc.
At room temperature, most metals carry a very thin oxide layer as a result of the metal's reaction with oxygen in the atmosphere. Metals subjected to heating may well carry a heavier layer, or the layer may detach. For example, steel which has been hot-rolled has a complex oxide layer which is physically unstable but still has a protective value provided the steel remains in air and as long as the layer remains a continuous layer.
Zinc in air carries a fairly protective film of zinc oxide, which increases in thickness very slowly. Aluminium carries a thin, highly protective oxide layer.
Dry corrosion may seem unlikely. However, it is worth remembering some corrosion takes place even under completely dry conditions. It needs removing before applying any form of protective coating.
"Wet corrosion" takes place in wet environments, i.e. where relative humidity exceeds 60%. These environments can be neutral, acid or alkaline.
There may be uniform destruction of the metal, e.g. oxidation or, localised destruction, i.e. pitting and stress corrosion. The destruction can be concentrated at areas adjacent to a more noble metal or, at points where the oxygen supply is limited.
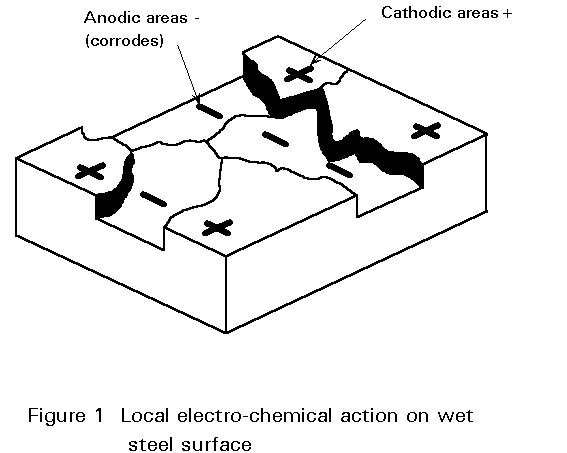
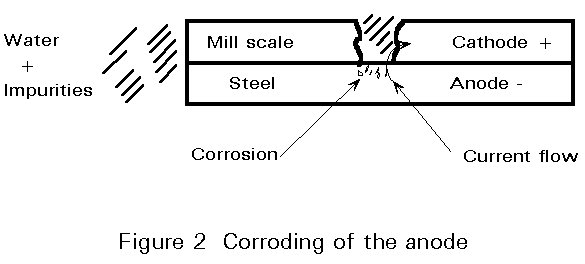
Wet corrosion is electro-chemical. When a metal is immersed in a conductive liquid (sulphur compounds in water in an industrial atmosphere or sodium chloride in water in a marine environment) some areas have a different electrical resistance from the rest of the surface (Figure 1). A "positive" electric current flows from the negative (-) anode to the positive (+) cathode areas and this leads to the dissolving or "corroding" of the anode (Figure 2). In other words, a "corrosion cell" is, broadly speaking, the same as a car battery.

In corrosion prevention literature the terms "galvanic " and the "galvanic series" are frequently used.
Galvanic corrosion is the destruction of the less-noble of a pair of metals joined together, e.g. in sea water zinc is less noble than mild steel and the zinc wastes away rapidly.
The galvanic series is a list of metals arranged in order of their corrosion potential with the most easily corroded at the top and the least active at the bottom. The simplified listing which follows shows why aluminium and zinc are used as coatings to protect mild steel and, stainless steel to replace it in certain circumstances.
Aluminium
Zinc
Iron
Mild Steel
Stainless Steel
Lead
Copper
Silver
Gold
Platinum
The most active metals, e.g. zinc and aluminium, are described as having negative electrical potentials. They may be referred to as anodic. The least active, e.g. gold and platinum are referred to as noble or, cathodic.
When dissimilar metals are connected in the presence of an electrolyte the more noble (cathodic) one tends to be protected while the more active (anodic or negative) corrodes rapidly.
As the potential difference between two dissimilar metals increases so does the possibility for galvanic corrosion.
To sum up, the corrosion of metals is simply a reversion of their extraction process. 90% of marine and industrial corrosion is electro-chemical and the reaction approximates to that which takes place in a car battery.
Basically steel is an alloy iron and carbon, other elements being added depending upon the processing method and the final performance required. Structural steels (medium carbon steels) contain 0,12% to 0,24% carbon. It was noted above that iron ore is iron oxide and rust is iron oxide plus chemically bonded water. Steel is man-made and unstable. It combines readily with oxygen and water, producing an iron oxide not unlike the original iron ore prior to refining.
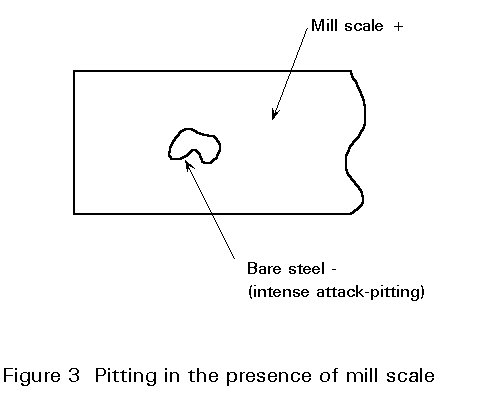
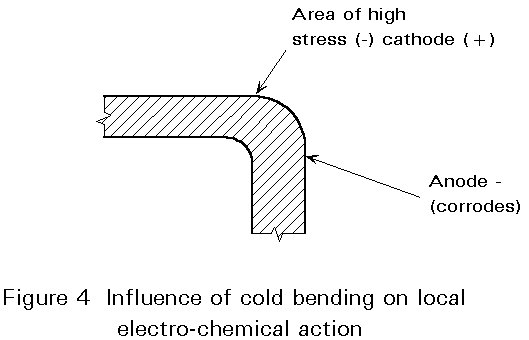
Electro-chemical corrosion can be highly concentrated at certain points. If this occurs, a high rate of destruction at points representing no more than 1% of the total surface area can destroy the usefulness of a steel component. There are a number of reasons why local corrosion can be so concentrated:
The oxide layer on hot-rolled mild steel is called mill scale. As noted earlier it is physically unstable. It is a separate entity from the steel in much the same way as is a coat of paint.
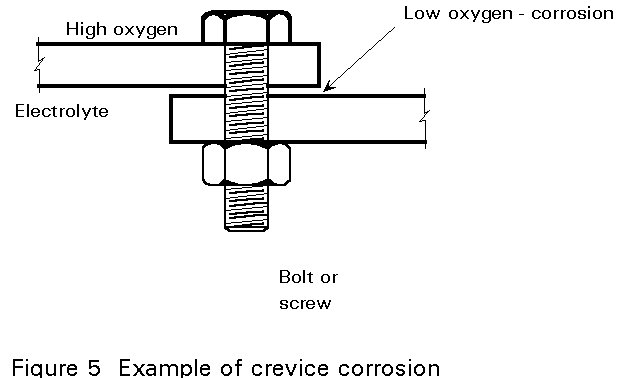
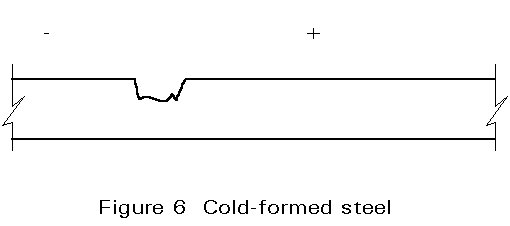
There are some fundamental practical considerations.
Corrosion is most likely to occur when one or more of the following is present:
Before deciding how to protect any steel, one must answer the question "From what?". Table 1 gives general environments. Answering the following will help produce the answer:
a) What is the general environment?
b) Is the environment likely to change in the foreseeable future? If it is, what is the cause and how will it alter the general environment?
c) Is there local pollution, e.g. sulphur dioxide, which could make the environment more aggressive than is first apparent?
d) In terms of environment must the project be divided into different parts when determining the protective system(s) or can the worst case be applied everywhere to simplify matters?
e) What special conditions apply, e.g., watersplash, residual pools, which may exclude the use of specific coatings?
f) Can the protective system chosen be maintained effectively and economically throughout the required life of the structure/plant?
The most practical way to protect steel is by applying another coat either to act as an anode (i.e. dissolving in preference to steel), as a barrier, or both. The common protective coatings are paints, hot dip galvanising, zinc or aluminium metal spray and any of the last three overcoated with paints. Their main features are summarised in Appendix 1. They are discussed in Lecture 4A.2.
Surface preparation is a major influence in determining the protective value of a coating system. For metallic coatings it is usually an integral part of the manufacturing process and is included in the relevant national and European standards. For paints the type and standard of surface preparation should be specified as a part of the protective coating treatment.
The importance of removing mill scale is well established. The methods used for this purpose and, to remove rust, etc. are as follows:
Weathering
Because the layer of mill scale is a physically unstable separate entity which breaks up before it leaves the rolling mills, the practice of "leaving the steel to weather" still exists. Unfortunately, the duration of weathering necessary to remove the scale from structural steel depends on the local climate, the type of mill scale, its thickness, the shape and thickness of the section and, when weathering takes place after the steel is erected, by the position of the individual structure. The time necessary to remove 90% of mill scale from 9mm thick plates of mild steel varies from about six months in industrial atmospheres in Europe to more than five years in certain overseas areas. Even in the most aggressive environments complete descaling by weathering of a structure could not be guaranteed within a year. Remember too it is in the aggressive marine or industrial environments where the chemical impurities which dissolve in water to form good electrolytes are found!
Weathering is not recommended as a preparatory method.
Chipping, Scraping and Wire Brushing
Chipping, scraping and wire brushing are by far the least effective methods. They do not remove deep-seated rust, or tightly adhering mill scale.
Mechanical wire brushes and scrapers give better results than manual tools, but the standards achieved are inferior to the alternative methods below.
Pneumatic descaling pistols
A bunch of hardened steel needles is held loosely in the collar of the pistol. Operated by compressed air, the needles move backwards and forwards in the collar to pound, literally, the surface. This preparatory tool is particularly useful around nuts, bolts and rivet heads. It is extremely slow to use and will not remove deep-seated rust or thin mill scale.
Flame cleaning
An intensely hot oxy-acetylene flame is played on the surface. Differential expansion causes the mill scale to detach. The process is extremely slow, but it can be effective. It will nor remove tight mill scale. It cannot be used upon steel which is less than 5mm thick, because it may cause buckling. In addition, it may "burn-in" chemicals deposited on the surface causing premature paint failures. Its use as a preparatory method is diminishing.
Acid pickling
This is a factory process for use on new steel before erection. The steel is immersed in hot sulphuric or hydrochloric acid; after rinsing it may be dipped in a weak phosphoric acid solution which deposits a thin crystaline phosphate coating upon the surface of the steel. This coating gives a very low level of protection against corrosion for a limited period. This form of acid pickling is one of the cheapest and most effective ways of removing all mill scale and rust. It is not a satisfactory form of surface preparation for use beneath sophisticated primers.
Cold site-applied pickling solutions are not effective.
Abrasive blast cleaning
This is an extremely effective method of removing mill scale and rust. Chilled iron grit or shot is projected by air or, centifrugally from a wheel. When carried out in the factory it is a relatively cheap process, but it can be expensive on site. It is not always practicable on erected steel.
Properly undertaken the process leaves the steel in an excellent condition to receive paint systems and metal spray. Its advantage is the profile which is produced and upon which the applied coating "keys".
Other Considerations
Surface Cleanliness in terms of how effectively mill scale and rust have been removed is covered by the pictorial illustrations in ISO 8501 [1].
Surface Roughness is important in respect of the profile produced by abrasive blasting, which roughens the surface of steel. ISO 8503 Part 1 specifies comparator panels as a means of specifying surface roughness [2]. These panels are used to make visual and tactile comparisons with the blast-cleaned surface.
Chemical Cleanliness too often is confused with surface cleanliness by the specifier. Rust formed in industrial or marine environments can contain soluble salts. These salts are often found in corrosion pits and are rarely removed by abrasive blast cleaning and never by mechanical or hand cleaning. If overcoated they lead to rapid coating failure. Wet abrasive blast cleaning or washing with potable water are both used as a means of cleaning chemically contaminated steelwork. Unfortunately standard methods are not available for the qualitative or semi-quantitative determination of the levels of chlorides, sulphates or soluble iron salts on freshly blast-cleaned steel. Methods are being developed and may be included in ISO 8502 and 8504 [3, 4].
The phenomena of galvanic corrosion and the galvanic series are the basis of Cathodic Protection (CP), a system in which the structure to be protected is made the cathode. For example if iron and copper are connected in sea water the iron corrodes; connect a piece of zinc into the system and a current flows from the zinc to the iron and copper and turns the iron into a cathode, i.e. the non-corroding pole in the electrochemical cell.
Cathodic protection using sacrificial anodes is established for the protection of steelwork under immersed conditions. For large installations, e.g. marine jetties, an "impressed current" system is often used. In this system the anode is inert, e.g. graphite or titanium, and a DC supply provides the voltage.
Setting up a cathodic protection system on an immersed or semi-immersed structure requires expert advice. There are however three points to remember when considering a CP system.
a. To do their job, sacrificial anodes must corrode! They require regular inspection to ensure they are replaced before they disappear completely.
b. There must be sufficient anodes to give the correct current density over the complete surface to be protected.
c. In systems using an external DC supply the polarity of the electrical connection is vital. Reversed connections can cause extremely rapid corrosion of the item the system is supposed to protect!
In buildings and plant stainless steels can be used for interior decoration, facades, cladding, fasteners and equipment. The resistance to atmospheric corrosion associated with stainless steel stems largely from its chromium content which helps to form a thin protective oxide layer which is also aesthetically pleasing.
There are three types of stainless steel currently used in buildings and it must be said that in-service failures are more often due to mis-specification than to inherent weaknesses in the products (see Lectures 18). Stainless steels do corrode!
The three grades have different mechanical properties which affect forming, welding and performance in service. To make the best choice for a particular application, the environment, the likely frequency of cleaning, e.g. by rainwater, the mechanical properties needed during fabrication, and the required performance in service need to be known.
Stainless steel components are expensive (x 10 the cost of carbon steel) and merit careful consideration before specifying to ensure their full potential is realised.
These steels contain only 1-2% of alloying additions, e.g. copper, chromium, nickel, phosphorus. They can be more corrosion resistance than similar unalloyed steels. But the protective coating only forms when the steel is subject to regular wetting and during cycles. Wet interiors, immersed or buried conditions are unsuitable environments in which to use weathering steels. Undoubtedly specialist advice from the steel industry is required before this type of steel is specified. There are a number of general considerations:
a. for long life a corrosion allowance must be considered because the actual loss varies with the environment.
b. crevices and other water/dirt traps must be designed out.
c. as the steel begins to weather iron hydroxides may run to adjacent surfaces and cause straining.
d. fasteners should be made of weathering steel.
e. specific low alloy welding rods are needed.
f. to obtain even weathering, blasting overall may be necessary.
g. these steels are unsuitable for use in marine and aggressive industrial environments.
ISO 8500 series Preparation of steel substrates before the application of paints and related products.
[1] ISO 8501 Visual assessment of surface cleanliness
- Part 1 Rust grades and preparation grades of uncoated steel substrates and of steel substrates after overall removal of previous coatings.
- Part 2* Preparation grades of previously coated steel substrates after localized removal of previous coatings.
[2] ISO 8502 Tests for the assessment of surface cleanliness.
- Part 1 Field tests for soluble iron corrosion products.
- Part 2 Laboratory determination of chloride on clean surfaces.
- Part 3 Assessment of dust on steel surfaces prepared for painting (pressure sensitive tape method).
- Part 4* Guidance on the estimation of the probability of condensation prior to paint application.
[3] ISO 8503 Surface roughness characteristics of blast-cleaned substrates.
- Part 1 Specifications and definitions for ISO surface profile comparators for the assessment of abrasive blast-cleaned surfaces.
- Part 2 Methods for the grading of surface profile of abrasive blast-cleaned steel. Comparator procedures.
- Part 3 Method for the calibration of ISO surface profile comparators and for the determination of surface profile - Focusing microscope procedure.
- Part 4 Method for the calibration of ISO surface profile comparators and for the determination of surface profile - Stylus instrument procedure.
[4] ISO 8504 Surface preparation methods.
- Part 1 General principles.
- Part 2 Abrasion blast-cleaning.
- Part 3 Hand and power tool cleaning.
[5] ISO 12944* Protective paint systems for steel structures
- Part 1 General Introduction.
- Part 2 Classification of Environments.
- Part 3 Types of Surface and Surface Preparation.
- Part 4 Classification and Definitions of Paint Systems and Related Products.
- Part 5 Performance Testing.
- Part 6 Workmanship.
- Part 7 Design.
- Part 8 Guidance for Developing Specification for New Work and Maintenance.
*
In course of preparation
APPENDIX 1
Characteristics of Paint and Metal Coatings
The features of hot dip galvanizing and metal spray to be considered:
a. Life predictable if the environment is accurately assessed.
b. Single application systems (plus sealing in some cases).
c. Short in-shop time.
d. Good abrasion resistance.
e. Sacrificial protection of steel provided at areas of damage.
f. Good corrosion resistance.
g. Should not be used unpainted outside the pH range of 5-12 for zinc and 4-9 for aluminium, or, where the metal is subject to direct attack by specific chemicals.
Hot dip galvanizing has additional features:
h. The alloying action provides a good metallurgical bond.
i. Full coating on sharp corners and edges. Thickness is influenced by the composition of the steel.
j. Adhesion problems can occur between the zinc and subsequently applied paint. These problems can be overcome by the use of special pretreatments, primers or specially formulated direct application paints.
Additional features for metal spraying:
k. Can be site applied.
l. Coating thickness can be built up as desired.
m. Virtually any size of structure and plant can be coated.
n. Often a better substrate for paint systems than hot dip galvanizing.
o. Should be sealed if to be over-painted.
p. Irrespective of environment aluminium-sprayed metal is best supplied sealed ex-works.
q. Where exposure is unlikely to be aggressive, zinc sprayed steel need not be sealed. If there is any likelihood it will need painting or sealing at a later date, then it should be sealed initially to prevent the formation of zinc corrosion products.
The main features of paint systems are:
a. Predictable life if the environment is accurately assessed.
b. Does not affect the mechanical properties of steel.
c. Suitable for shop and site use.
d. Can be applied to complex structures and plant.
e. Systems are available to protect against most environments and conditions.
f. Painting facilities are widely available.
g. Most paints are easy to repair and maintain.
h. Wide colour ranges are available for safety and decoration.
i. High performance paints require high standards of surface preparation (usually abrasive blast cleaning) and can be intolerant of poor painting conditions.