ESDEP WG 4A
PROTECTION: CORROSION
To introduce students to the protection of structures, pipes, etc. offshore and in the ground.
Lecture 4A.1: General Corrosion
Lecture 4A.2: Factors Governing Protection of Steelwork
Lecture 4A.3: Corrosion in Buildings
Lecture 4A.4: Corrosion Protection of Bridges
This lecture covers the systems available to protect fixed and mobile offshore structures. It deals with sheet piling, corrosion in soils, electrical methods of corrosion control and, very briefly, bacterial activity and its influence on corrosion rates.
The North sea is far more hostile than the Texan and Arabian Gulfs. The technology used in the warmer climates has had to be adapted and modified to meet the colder climatic conditions both in coastal construction sites and, offshore in the North Sea.
The difficulty of repairing coatings or, worse still, replacing steel make it imperative that the coating system prevents loss of steel by corrosion.
Fixed offshore constructions are divided into three areas, underwater and tidal, the splash-zone and, decks.
Underwater and tidal zones
Experienced corrosion engineers are not always of the same opinion about the choice of a protective system for steel in these zones.
Some argue that the area below the lowest low waterline (LLW) is subject to mechanical damage during construction and hook up and: that no protective coating can be expected to survive for some 25 years in the sea. Therefore cathodic protection (sacrificial anodes or impressed current) is necessary to back up the coating systems. The argument then questions whether coatings are necessary, since cathodic protection is easily increased in capacity and can therefore provide total protection of the uncoated steel surface.
The opposing view is that even damaged coatings reduce impressed current amperage and anode consumption. Coatings will certainly improve corrosion protection in acute angles and in welded areas. It seems sensible therefore to at least balance the cost of coating steel against cathodic protection only.
There is no debate regarding the advantages of using surface coatings in the tidal zone and for up to 10 metres below lowest average tide (LAT). Increased steel thickness to allow for corrosion in this highly aggressive area may be provided, but cathodic protection combined with coatings is always used.
The splash zone and jetties
These zones can be well protected by the coatings systems which are effective in the tidal zone. The undersides of support decks and soffit areas in general also come into this category.
Any system chosen for service in this environment must have very good resistance to water, rust creep and oil/chemical spillage. Coatings need to be suitable for patch repairs.
Decks
The decks on offshore platforms may require the protection of heavy duty coating systems because they are subjected to severe traffic, impact, and chemical and oil spillage. On jetties the decks are often an extension of the approach road and not vulnerable therefore. Where they are of steel, then the same considerations apply as are given to protecting offshore rigs.
Mobile offshore equipment
A wide range of items if involved, e.g. jack-up drilling rigs (for shallow water), self-propelled and semi-submersible drilling rigs, and supply ships. Service conditions vary with operating conditions and the type of equipment. All suffer severe impacts and abrasions resulting in the destruction of the protective coatings. Maintenance is difficult and is usually carried out at the same time as mechanical repairs and overhauls and during lay up periods. The coatings systems used to protect mobile offshore equipment are similar to those used on fixed offshore structures.
Paint Selection
Paint selection is greatly influenced by the likely weather conditions during construction and of course, maintenance. For new work, surface preparation (which is always abrasive blast cleaning) and the application of the first coats should be undertaken in shops where temperature and humidity can be controlled. It is impossible to complete the final assembly of most offshore structures under cover, while the sheer complexity of fabrication requires a great deal of spot repairs which can only be undertaken in the open. Although local abrasive blast cleaning is the normal preparatory method when dealing with fabrication damage, there are occasions when only power tool cleaning is possible. The products used for patch repairs must be capable of performing well (although inevitably at a reduced level) over such preparation and of application under exposed conditions, possibly in adverse weather. The finishes used on the complete structure are also required to perform well under similar conditions.
Typical Systems for Underwater, Tidal and Splash Zones:
New Works
Surface preparation in all cases is abrasive blast cleaning to Sa 2½, ISO 8501-1 [1].
Possible primers are one coat of:
2-pack zinc silicate @ 75mm
or 2-pack zinc epoxy @ 35mm
or 2-pack epoxy holding primer @ 50mm.
The common finishing coat would be:
2-pack coal tar epoxy, amine aduct cured,
volume solids < 60% applied by airless
spray at 200mm per coat.
Maintenance in service
The maintenance painting of fixed offshore structures, jetties, etc. is unlikely to be possible for more than 4-5 months during the spring and summer (the so-called "weather window"). Inevitably work is halted due to poor weather, when the last coat is exposed to complete or nearly complete immersion, salt spray, etc. Thus, ideally each coat must be as resistant to the environment as the final finish.
It is feasible to repair underwater areas using water displacing solvent free epoxy coatings. Since these coatings would be applied by divers, the problems are obvious. It is usual therefore to rely instead upon cathodic protection to prevent corrosion at breaks in the coating.
Damage in the tidal or splash zones must be repaired because cathodic protection is not effective for more than 0,2m above lowest waterline.
Typical Systems for Use above the Splash Zone:
New Works
The choice of primer is from those suitable for the underwater, tidal, splash zones described above. Surface preparation is abrasive blast cleaning to Sa 2½, ISO8501-1.
The finishing system is likely to consist of high build 2-pack polyamide cured epoxy intermediate coats applied at dry film thicknesses (dft) of 100-150mm per coat. Usually two coats are applied. The first may be pigmented with micaceous iron oxide to increase build on edges. The finish coat of circa 50mm dft is either a 2-pack paint described as "urethane" or "polyurethane" and probably based on an isocyanate cured acrylic resin or a 1-pack moisture curing urethane, i.e. a material which reacts with atmospheric moisture, producing an end product with similar characteristics to 2-pack varieties. The final coat is usually modified to ensure it can be over-coated using fabrication and maintenance painting without the need to first sweep blast to obtain adhesion. The product description in the manufacturer's technical literature normally carries the word "recoatable". Where appearance is not of prime importance, it is possible to use a zinc silicate primer (75mm dft) followed by two coats of a 2-pack high build polyamide cured epoxy applied at circa 150mm dft per coat.
Maintenance
Maintenance usually requires blasting to Sa 2½ followed by the application of the original system. The treatment may be over all or spot depending upon whether the coating breakdown is severe or isolated.
Where surface preparation utilises water abrasive blast cleaning, then special primers are required, e.g. 1-pack zinc rich epoxy, moisture displacing modifications.
If blast cleaning is impossible, power tool cleaning to the St2, ISO 8501-1 followed by priming with a 2-pack epoxy aluminium pigmented "mastic" before finishing as noted above is an alternative. Its use should be limited to minor repairs.
Typical Systems for Decks:
New Works
Decks require very heavy duty systems which are required to be "non-skid" as well as resistant to the rigorous conditions noted elsewhere. Solvent free 2-pack epoxy systems are available for direct application in one coat of 3000mm to blasted surfaces (Sa 2½). These coatings can contain flint to prevent them becoming slippery in service. Non-sparking alternatives are available for use in flame-free zones.
Normal heavy duty 2-pack epoxy coatings of the type used in the splash zone are also used in two coat applications (total dft 200+mm) over zinc silicate, zinc rich or zinc-less 2-pack epoxy primers.
Maintenance
Maintenance is usually blasting to Sa 2½ followed by the re-application of the original systems, either over all or in localised areas of damage. If for some reason blasting is not possible, then power tool cleaning to St3, ISO 8501-1 may be acceptable for the more normal coating system, i.e. 2-pack epoxy paint system at 200mm. The heavy duty coating demands a high standard of abrasive blast cleaning. Surface preparation for maintenance must be the same as the original.
The steel sections used for steel sheet piling provide the maximum strength and durability (consistent with good driving properties) at the lowest weight which good design can achieve. Sheet piling is used in both permanent and temporary works. Typical applications are sea defences, land reclamation, quays, and coffer dams. Sheet piling can be spliced where lengths of more than 30m are required and, although sheet piling is usually associated with "straight line" constructions, e.g. quay walls, the sections can be used to form many shapes, including complete circles.
The effective life of unprotected piling, either mild steel or high yield steel (e.g. grades S235, S275 and S355 to EN 10025) depends upon the stresses imposed in service and the corrosion rate. The potential life of sections can vary from more than 120 years when exposed to an atmosphere with a mean corrosion rate of 0,05mm/year to circa 80 years in a splash zone with a mean of 0,09mm/year assuming only one face of the piling is exposed. Where both sides of a sheet piling structure are exposed to the atmosphere for example, the effective life can be reduced by some 30%.
Many environments in which steel piling is used have low corrosion rates. Piles driven into undisturbed soils are quoted as corroding at no more than 0,03mm/year. In this situation protection is superfluous. Conversely the rate in a splash zone may be as high as 0,15mm/year. If the imposed stresses are also high, then measures to increase the life of the structure are needed. Replacing mild steel with high yield steel is a possibility, as is the inclusion of additional steel thickness. Cathodic protection may be appropriate. High on the list of protective methods are paints.
Protective coatings should meet certain requirements:
These properties are necessary to ensure a speedy throughput in the shops where the initial coating is applied.
Additionally the coating must:
A number of painting systems are available to the specifier, who should consider only those which were designed for this particular end use. One specialist, British Steel, advocates the use of only two alternatives, both specifically designed for piling.
The first system is a high build two pack epoxy/pitch which is isocyanate cured. This is applied to abrasive blast cleaned surfaces of Sa 2½ quality at up to 400mm in one or two coats. Typical end uses are piers, jetties, harbour walls and bearing piles in corrosive soils. The typical time to first maintenance under severe exposure conditions is quoted as 15 years. Maintenance is either blasting followed by the airless spray application of the original coating in one coat at 400mm or two coats of what is in fact the second system suggested as suitable for piling protection.
The second coating system is described as a tar-vinyl. This coating is a pitch modified with specific vinyl resins which impart a measure of elasticity. The one part product dries soley by solvent evaporation, which makes it particularly suited to site use. It can be applied at up to 150mm per coat. Typical uses are canal and river walls and, general structures not exposed in aggressive environments. Although abrasive blast cleaning to Sa 2½ is preferred, this material can be used effectively over power tool prepared steel St2 or 3, ISO 8501-1. Maintenance requires the application of the original material to the initial dry film thickness or it can be overcoated with other high build pitch solutions.
Only the isocyanate cured epoxy pitch is suitable for use on catholically protected structures.
Corrosion in soils is similar to corrosion in water. It requires the presence of an electrolyte and circumstances which produce anodic and cathodic areas in the steel or iron.
For soil conditions to cause corrosion there must be:
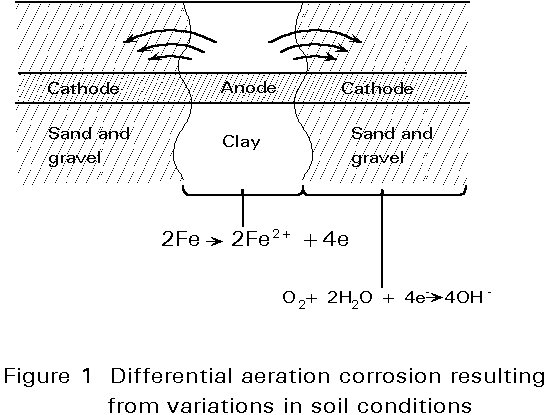
Buried steel structures are rarely exposed to conditions in which the electrolyte is acidic enough to support corrosion without the presence of oxygen to maintain the process. Corrosion usually requires the soil to be pervious to air, with variations in soil conditions giving rise to differential aeration and the creation of cathodic and anodic areas (see Figure 1). An exception is in neutral, waterlogged clay; although oxygen cannot penetrate the soil, corrosion occurs by a sulphate-reducing bacteria present in the soil (see Section 5).

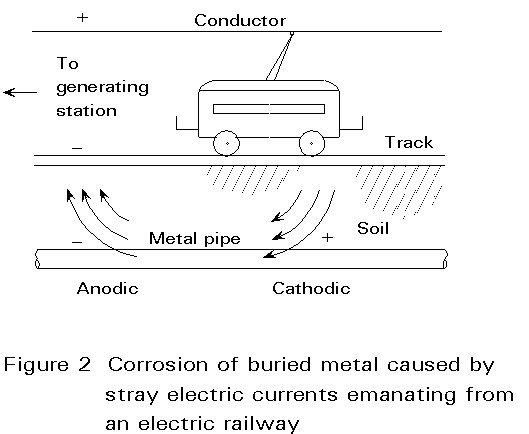
Stray electric current can cause severe corrosion, the buried metal providing a low resistance path. The metal acts as a cathode at the point where the positive current enters and becomes the anode where it leaves the metal. Figure 2 illustrates the problem. The effect is worse with direct current.
The methods of controlling corrosion in soils are limited. Where the soil is acid, then pipelines can be made less vulnerable by back-filling trenches with limestone chippings which changes the pH. This method is unlikely to be suitable for structures or piles, where the soil is required to be undisturbed for engineering purposes. In many cases the only effective answer is to re-route the pipeline or re-site the structure.
Traditionally, buried structures have been protected by coating with paints based on coal tar, pitch or bitumen reinforced with a resin. Today, popular resins for such purposes are epoxy, vinyl or urethane derivatives, i.e. the coatings discussed above as suitable for protecting immersed steel or piling in soil. In the case of buried pipes other coatings, e.g epoxy powder, three layer polyethylene or wrappings, may be more appropriate.
With all buried steel and iron, cathodic protection should be considered as a means of reinforcing the performance of the coating.
The best known method is cathodic protection but there are also other methods, i.e. anodic protection and electrical insulation.
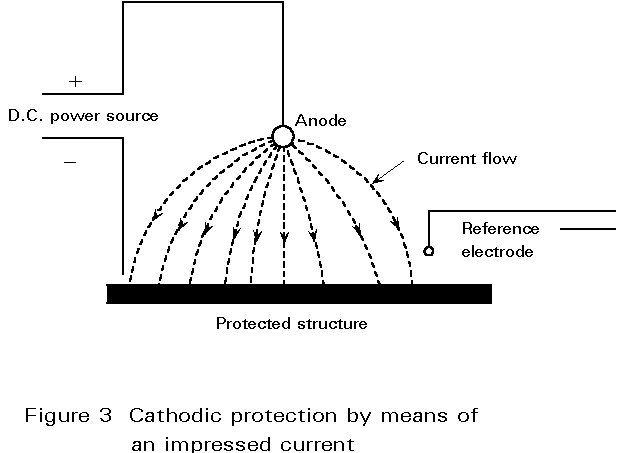
Cathodic protection relies upon the fact that, if the complete surface of a metal can be made cathodic by using an external electrode, then corrosion will not occur. Figure 3 illustrates the use of an impressed current system. Usually a low voltage direct current is the external current which confers protection. The positive terminal of the current source is connected to an auxiliary anode which is located away from the structure requiring protection. The structure itself is then connection to the negative terminal and current flows from the anode to the protected structure. The latter is usually coated or wrapped because the current required to protect an uncoated structure would be too high to make the method economic. Thus, the coating or wrapping is the prime defence with cathodic protection dealing with breaks in the coating.
Sacrificial anodes (zinc, aluminium, magnesium alloys) are also used (Figure 4). In this case the auxiliary electrode is made of a metal more active than the one requiring protection. It becomes the anode in the corrosion cell when connected to the item requiring protection, which becomes the cathode. No external current is required, but protection ceases if the anode corrodes completely.

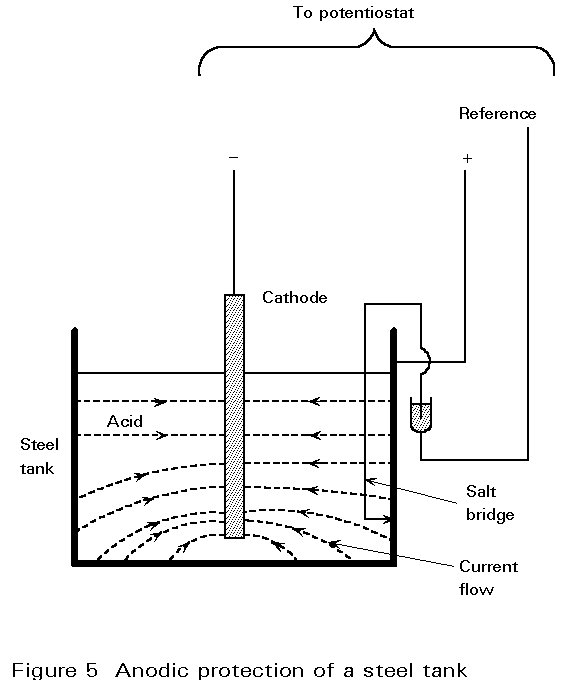
Anodic protection requires a "passive" layer to form and protect the metal from corrosion. An external current is applied in the opposite direction to that for a cathodic system. It drains electrons from the surface, raising the potential of the anode to a value at which the metal becomes passive (see Figure 5). The current density required is high, but once passivity is attained only a very small current is required to run the system. A further attraction is the high "throwing" power of the current which reaches areas remote from the cathode. The system is potentially useful inside complicated tanks and long pipelines.
The liquid being handled must lend itself to passivation, e.g. sulphuric acid. The current and the environment require careful monitoring to ensure that the correct conditions are maintained. If they are not, rapid corrosion occurs.
Electrical insulation increases the resistance at some part of a corrosion cell, reducing the current flow and therefore the corrosion rate. For example, the electrical resistivity of demineralised water is much greater than that of a salt solution and the rate of corrosion in the former is correspondingly lower.
Re-routing pipes to avoid low resistivity corrosive soils reduces the corrosion risk. Laying pipes through soils of differing resistivities may set up anodic areas in the metal in low resistivity soil and cathodic regions in high resistivity soil, creating corrosion cells.
Such "long line" currents are prevented by inserting insulating joints between sections of pipes.
Finally, insulating dissimilar metals from each other, e.g. aluminium cladding sheets from the supporting steel, eliminates the risks of galvanic corrosion.
Although detailed explanations are beyond the scope of this lecture, engineers and architects should be aware of the effects of aerobic and anaerobic organisms in promoting the corrosion of steel or ironwork immersed in water or buried in the ground. In practical terms expert advice should be obtained when dealing with any project where the environment is likely to promote the growth of bacteria.
Aerobic organisms are those requiring free oxygen to prosper and anaerobic ones are organisms which require no oxygen.
In the field of corrosion, sulphate reducing bacterial (SRB) are probably the best known; they are certainly the most destructive. In our predominantly aerobic environment they are dormant because they are strictly anaerobic organisms. As the oxygen level in an environment decreases, e.g. in stagnant water areas on a jetty at low tide, the SRB become active. As the cells increase by-products are produced which initiate anaerobic corrosion. Their metabolism requires the reduction of sulphate molecules to water, releasing free sulphide which reacts with hydrogen to form an extremely corrosive gas, hydrogen sulphide. This process not only depresses the oxygen level, encouraging further growth, but reacts with the iron and steel.